Essential Questions:
- What role does energy play in chemical reactions?
- How does structure impact the properties and behavior of atoms, molecules, and compounds?
|
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
|---|---|---|---|---|
| Title |
Subatomic Symphony: Unraveling Atomic Structure |
The Periodic Puzzle: Uncovering Periodic Trends and Exploring Chemical Nomenclature |
A Balancing Act: Exploring Chemical Equations and Thermochemistry |
Mixing Matters: Exploring Solutions |
|
Image |
|
 |
 |
|
|
Focus o the Story |
How has our understanding of the atom evolved over time?We begin by looking at how chemistry surrounds us in our everyday lives. We will then look at the structure of the atom, the smallest unit of matter, which will be a part of our study throughout the whole course. |
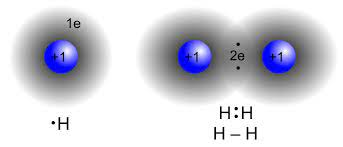
How do intermolecular forces and the type of bonding determine physical and chemical properties of a compound? Next we will look at how the position of an element on the periodic table will help us to predict how the atom will behave. We will learn how atoms form bonds based on how their electrons interact. |
How is stoichiometry useful? Next, we will learn how chemical equations are used to describe the number and arrangement of atoms in chemical reactions. We will also use a type of math for chemistry to help us describe the composition and quantities in a chemical reaction. |
How are gas behaviors predicted from variables and mathematical relationships? |
|
Transfer Goals |
|
|
|
|
|
Learning Targets |
|
|
|
|